By Eduardo Simões and Rodrigo Viga Gaier
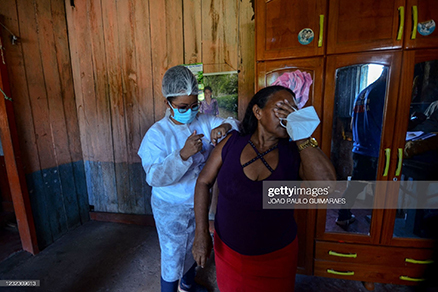
SAO PAULO/RIO DE JANEIRO, Brazil, May, 11, 2021 (Reuters) – Brazilian states halted vaccination of pregnant women on Tuesday after a death in Rio de Janeiro led health regulator Anvisa to warn against the use of AstraZeneca’s COVID-19 vaccine for expecting mothers.
A pregnant woman in Rio de Janeiro died after receiving the AstraZeneca shot, according to state Health Secretary Alexandre Chieppe, in a case authorities are still investigating.
Sao Paulo state suspended COVID-19 vaccination for pregnant women with risk factors and Rio state suspended immunization of all pregnant women. Both states cited the Anvisa recommendation as a reason for the decision.
Asked about the suspension, AstraZeneca said in a statement that pregnant women and those breastfeeding were excluded from clinical trials of its COVID-19 vaccine. Studies in animals did not produce direct or indirect evidence of harm regarding pregnancy or fetal development, the statement added.
The Health Ministry said it was investigating the case, newspaper Folha de São Paulo reported, citing a statement from the ministry late on Monday. The ministry did not immediately respond to a request for comment on Tuesday.
The AstraZeneca vaccine is produced and distributed in Brazil via a partnership with public health institute Fiocruz.
Fiocruz did not immediately respond to requests for comment.
(Reporting by Eduardo Simões in São Paulo and Rodrigo Viga in Rio de Janeiro Writing by Ana Mano Editing by Brad Haynes, Chizu Nomiyama and Jonathan Oatis)